Abstract
Introduction: Outcomes for children and adolescents/young adults (AYAs) with relapsed and/or refractory (r/r) acute myeloid leukemia (AML) remain poor despite maximal intensification of cytotoxic chemotherapy. Immune-based therapies, including bispecific antibodies, have potential to overcome chemoresistance and induce remission in patients with hematologic malignancies. We previously reported that highest expression of the CD123 cell surface antigen in pediatric AML is associated with inferior clinical outcomes (Lamble et al JCO 2022), providing rationale for immunotherapeutic targeting. Safety and activity of the CD123xCD3 bispecific antibody flotetuzumab (MacroGenics) was recently reported in adult patients with r/r AML via a phase 1/2 study (Uy et al Blood 2021).
Methods: The Children's Oncology Group (COG) PEPN1812 phase 1 trial (NCT04158739) tested the safety and tolerability of flotetuzumab in children and AYAs with second or greater r/r AML via a rolling six design. Subjects aged 1-30 years weighing ≥17 kg (due to vehicle formulation requirements) were eligible to receive up to 6 cycles of flotetuzumab. The primary aim of the study was to estimate the maximum tolerated dose (MTD) and/or recommended phase 2 dose (RP2D) of flotetuzumab in pediatric patients with r/r AML. Secondary aims included pharmacokinetic, pharmacodynamic, and immunogenicity characterization and assessment of preliminary clinical activity. Hematologic and non-hematologic dose-limiting toxicities (DLTs) were measured in subjects who received ≥80% of flotetuzumab dosing in cycle 1. Best clinical response was determined after up to 4 cycles.Subjects received flotetuzumab via continuous intravenous infusion for 28 days/cycle with a 7-day 'step-up' during Cycle 1. Three dose levels (DL) were planned; the initial goal DL was 500 ng/kg/day (DL1), with one de-escalation (300 ng/kg/day, DL-1) and one escalation (700 ng/kg/day, DL2). All subjects received pre-medication during 'step-up' dosing to mitigate infusion related reactions and cytokine release syndrome (CRS).
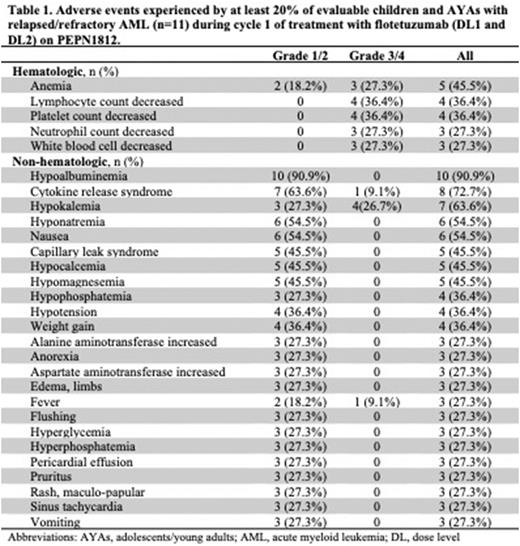
Results: Sixteen subjects (ages: 3-19 years) with r/r AML were enrolled from January 2020 to May 2021 with 15 subjects receiving at least one dose. One subject withdrew consent prior to treatment due to medical comorbidities. Amongst treated subjects, 7 had primary chemorefractory AML, 4 were in first relapse after hematopoietic stem cell transplantation (HSCT), and 4 were multiply-relapsed after HSCT. Seven subjects were treated at DL1 with 6 evaluable for toxicity; 8 were treated at DL2 with 5 evaluable for toxicity. While CRS and capillary leak syndrome (CLS) were frequently observed adverse events (AEs), ≥grade 3 toxicity occurred in a minority of subjects (Table 1). Other common AEs included hypoalbuminemia, hypokalemia and nausea. One subject treated at DL1 experienced a DLT of grade 4 CRS. A DLT of grade 3 creatinine elevation occurred in 1 subject treated at DL2. One subject treated at DL2 who achieved a complete remission (CR) after cycle 1 had grade 3 CLS and grade 3 CRS during cycle 2, necessitating drug interruption until symptom resolution. The subject restarted flotetuzumab with DL1 step-up dosing 9 days following the event and clinical evidence of CRS and CLS did not recur. However, the patient experienced a grade 5 cardiorespiratory event following routine procedural sedation at end-cycle 2. Autopsy showed multi-organ infiltration with CD3+ lymphocytes and cardiac myocyte injury deemed probably related to flotetuzumab. Based on DL2 toxicity, DL1 was determined to be the RP2D even though the study criteria for defining the MTD were not met. Three subjects had a clinical response, including 1 CR with incomplete blood count recovery (DL1), 1 CR with partial platelet count recovery (DL2), and 1 partial response (PR, DL1). All other subjects experienced stable or progressive disease.
Conclusions: We report the safety, RP2D, and preliminary activity of the CD123xCD3 bispecific antibody flotetuzumab in children with r/r AML. Despite the frequent occurrence of mild CRS and CLS, flotetuzumab was determined to be safe and tolerable at the RP2D of 500 ng/kg/day (DL1) with an overall response rate (CR + PR) of 20% in children and AYAs with r/r AML. This study supports the potential of CD123-targeting immunotherapies in pediatric patients with AML and provides rationale for future phase 2/3 investigation.
Disclosures
Tasian:Incyte Corporation: Research Funding; Kura Oncology: Consultancy, Research Funding; Syndax Pharmaceuticals: Consultancy; bluebird bio: Consultancy; Beam Therapeutics: Research Funding; Aleta Biotherapeutics: Consultancy; GSK: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal